Derivatized cardo-polyetherketone anion exchange membranes for all-vanadium redox flow batteries†
Abstract
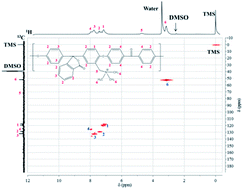
Cardo-polyetherketone (PEK-C) based anion exchange membranes (AEMs) were synthesized by chloromethylation of PEK-C followed by quaternization using trimethylamine (TMA). The ion exchange capacity (IEC) of the AEM was 1.4 ± 0.1 mmol g−1. The sulfate ion conductivity and vanadium(IV) permeability were 5.6 ± 0.5 mS cm−1 and 8.2 ± 0.2 × 10−9 cm2 s−1 at 30 °C. The chemical stability and mechanical integrity of the AEMs were investigated upon exposure to 1.5 M VO2+ solution by monitoring the ionic conductivity, ultimate tensile strength, elongation at break, and chemical structure over 1500 hours. 1-D and 2-D NMR spectroscopy confirmed the chemical stability of the AEM over this period. The ionic conductivity of the AEM decreased from 5.6 to 4.4 mS cm−1 over the first 48 hours but subsequently stabilized and was reversible, while the ultimate tensile strength and the elongation at break were reduced by ca. 35%. The PEK-C based AEMs were stable during operation in a vanadium redox flow battery (VRFB) for 100 hours of testing. The coulombic and energy efficiencies of the VRFB were 98% and 80%, respectively. Post-mortem analysis of the AEM using 1-D and 2-D NMR spectroscopy showed a 15% reduction in the number of quaternary ammonium groups in the AEM.

 Please wait while we load your content...
Please wait while we load your content...