Na3V2(PO4)3@C core–shell nanocomposites for rechargeable sodium-ion batteries†
Abstract
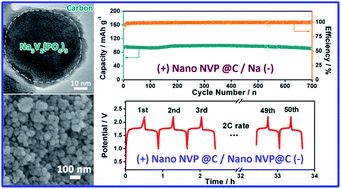
Na3V2(PO4)3 (NVP) is an attractive cathode material for sodium ion batteries due to its high theoretical energy density and stable three-dimensional (3D) NASICON structure. In this paper, a NVP@C core–shell nanocomposite has been synthesized through a hydrothermal assisted sol–gel method. Ascorbic acid and polyethylene glycol 400 (PEG-400) were synergistically used to control the particle growth and provide the surface coating of conductive carbon. The as-prepared nanocomposite was composed of a nanosized Na3V2(PO4)3 core with a typical size of ∼40 nm and a uniformly amorphous carbon shell with the thickness of a few nanometers. The electrode performance of the NVP@C core–shell nanocomposite as cathode for sodium ion batteries is investigated and compared with that of bare NVP and NVP/C. Among the samples examined, the NVP@C nanocomposite showed the best cycle life and rate capability. It rendered an initial capacity of 104.3 mA h g−1 at 0.5 C and 94.9 mA h g−1 at 5 C with a remarkable capacity retention of 96.1% after 700 cycles. Moreover, a full cell using the as-prepared nanocomposite as both the cathode and the anode active material has been successfully built, showing a reversible capacity of 90.9 mA h g−1 at 2 C with an output voltage of about 1.7 V and a specific energy density of about 154.5 W h kg−1. The enhanced electrode performance is attributed to the combination of particle downsizing and carbon coating, which can favor the migration of both electrons and ions.

 Please wait while we load your content...
Please wait while we load your content...