A pentakis-fused tetrathiafulvalene system extended by cyclohexene-1,4-diylidenes: a new positive electrode material for rechargeable batteries utilizing ten electron redox
Abstract
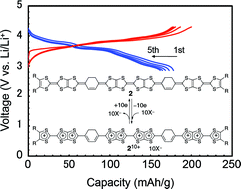
Pentakis-fused TTF derivatives extended with two cyclohexene-1,4-diylidenes (2a and 2b) have been successfully synthesized. Cyclic voltammetry of the tetrakis(2-ethylhexylthio) derivative (2b) has revealed that it exhibits five stages of the redox process to form 2b10+. A coin-type cell composed of a positive electrode incorporating the tetrakis(methylthio) derivative (2a) exhibits a discharge capacity of 196 mA h g−1, which corresponds to participation of a ten electron redox. It shows a high performance of energy density of 700 mW h g−1 and an output power density of 69 W g−1 with a relatively stable cycle-life performance (72% of the initial discharge capacity after 30 cycles).

 Please wait while we load your content...
Please wait while we load your content...