Fe3S4 (greigite) formation by vapor–solid reaction†
Abstract
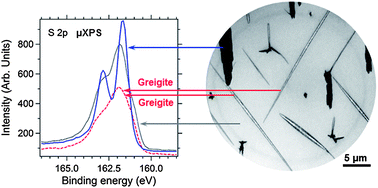
Fe3S4 (greigite), a ferrimagnetic compound widely encountered in nature and of increasing technical interest, is prepared by vapor–solid interaction. This is done in a laterally resolving ultrahigh vacuum multi-method instrument, which not only allows in situ analysis of the reaction product without influence of the environment but also follows its evolution during the reaction. The physical method employed here avoids formation of unwanted reaction products that are produced by the usual wet chemical synthesis methods. The resulting greigite crystals exhibit complex intergrowth, faceting and nanoparticle substructure and are somewhat distorted from a pure cubic symmetry. A new Curie temperature above 450 °C is established.

 Please wait while we load your content...
Please wait while we load your content...