Effect of iron-carbide formation on the number of active sites in Fe–N–C catalysts for the oxygen reduction reaction in acidic media†
Abstract
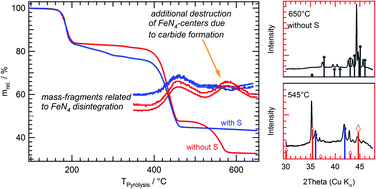
In this work Fe–N–C catalysts were prepared by the oxalate-supported pyrolysis of FeTMPPCl or H2TMPP either in the presence or absence of sulfur. The well-known enhancing effect of sulfur-addition on the oxygen reduction activity was confirmed for these porphyrin precursors. The pyrolysis process was monitored in situ by high-temperature X-ray diffraction under synchrotron radiation (HT-XRD) and thermogravimetry coupled with mass-spectroscopy (TG-MS). It was found that the beneficial effect of sulfur could be attributed to the prevention of iron-carbide formation during the heat-treatment process. In the case of pyrolysis of the sulfur-free precursors an excessive iron-carbide formation leads to disintegration of FeN4-centers, hence limiting the number of ORR active sites on the final catalyst. Physical characterization of the catalysts by bulk elemental analysis, X-ray diffraction (XRD), Raman and 57Fe Mößbauer spectroscopy confirmed the outcome from HT-XRD and TG-MS. It could be shown that the avoidance of carbide formation during pyrolysis represents a promising way to enhance the density of ORR active sites on those catalysts. This can be done either by sulfur-addition or the performance of an intermediate acid leaching. As iron carbide is often found as a by-product in the preparation of Fe–N–C catalysts this work gives some general strategies for enhancing the density of active sites enabling higher current densities.

 Please wait while we load your content...
Please wait while we load your content...