Structure–property relationships of symmetrical and asymmetrical azobenzene derivatives as gelators and their self-assemblies†
Abstract
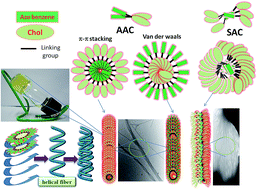
Two different series of symmetrical and asymmetrical azobenzenes containing terminal cholesteryl/adamantyl derivatives (SAC/SAA and AAC) with varying spacer lengths (alkyl chains) have been developed. The gelation and aggregation of these derivatives were studied relative to structural motifs, spacer lengths, solvent affinity, temperatures and light conditions. Among these derivatives, the cholesteryl derivatives that have short alkyl chains (<3) act as efficient gelators in a variety of solvents. However, the cholesteryl derivatives with longer alkyl chains (11 spacer) and adamantyl derivatives did not possess this ability. Self-assembled fibrous structures were constructed by gelators with short alkyl chains (<3), while flower-like structures were constructed by gelators with moderately longer alkyl chains (3–6) at their respective critical gelation concentrations (CGCs) according to SEM (Scanning Electron Microscopy) and TEM (Transmission Electron Microscopy) analyses. In some cases, a partial/weak gel was observed in different solvents, which exhibited uniform spherical nanoparticles at CGCs. These nanoparticles were further entangled to form interconnected fibrous structures when the concentration was increased above the CGC (according to the SEM and TEM analyses). Secondary forces (van der Waals/H-bonding) and π–π stacking played important roles in the aggregation of both series in the solvents according to variable temperature 1H-NMR analysis. The reversibility of sol–gel transitions by light was studied with respect to solvent affinity. This study revealed that reversible transitions were only observed in the non-polar solvents, as supported by the FTIR analysis of the gelators in the various solvents. The thermal and mesomorphic behaviors of the gelators by DSC (Differential Scanning Calorimetry) and POM (Polarized Optical Microscopy) analyses revealed that the chiral nematic (N*) and cholesteric mesophase (Ch*) were exhibited by only the short and longer alkyl chain cholesteryl derivatives, respectively. However, the cholesteryl derivative without a spacer (AAC0) did not exhibit any liquid crystalline phase but acted as an efficient gelator relative to the other gelators in this study.

 Please wait while we load your content...
Please wait while we load your content...