Evidence for equilibrium gels of valence-limited particles†
Abstract
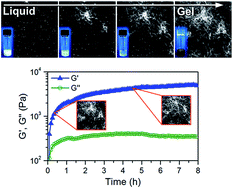
We explore the formation and structure of gels produced from solutions of the aromatic dipeptide derivative molecule fluorenylmethoxycarbonyl–diphenylalanine (Fmoc–FF) in dimethyl sulfoxide (DMSO). Mixing these solutions with water results in the self-assembly of Fmoc–FF molecules into space-filling fibrous networks, exhibiting mechanical properties characteristic of gels. Using confocal fluorescence microscopy, we observe the gel transition in situ and find that, upon the addition of water, the solution undergoes a rapid transition to a non-equilibrium state forming ∼2 μm spheres, followed by the formation of fibers 5–10 nm in diameter, nucleating at a sphere surface and expanding into the solution as the remaining spheres dissolve, extending the network. The gel aging process is associated with the network becoming increasingly uniform through apparent redissolution/reaggregation of the Fmoc–FF molecules, corresponding to the observed increase in the elastic modulus to a plateau value. We demonstrate that this increase in uniformity and elastic modulus can be expedited by controlling the temperature of the system, as well as that these gels are thermally reversible, further indicating that the system is in equilibrium in its fibrous network state. X-ray scattering information suggests that the packing of the molecules within a fiber is based on π–π stacking of β-sheets, consistent with models proposed in the literature for similar systems, implying that each particle (molecule) possesses a limited number of interaction sites. These observations provide experimental evidence that these low molecular weight gelator molecules can be considered valence-limited “patchy” particles, which associate at low enough temperature to form equilibrium gels.

 Please wait while we load your content...
Please wait while we load your content...