Thermal transitions in hydrated layer-by-layer assemblies observed using electrochemical impedance spectroscopy†
Abstract
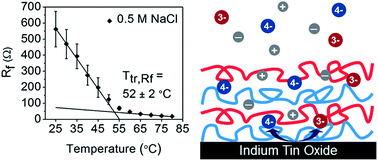
Layer-by-layer (LbL) assemblies have been of great interest due to their versatile functionality and ease of fabrication, but their response to temperature is not completely understood. It has been recently shown that hydrated LbL assemblies of poly(diallyldimethylammonium chloride) (PDAC) and poly(styrene sulfonate) (PSS) under go a thermal transition much like a “glass-melt” transition. This thermal transition is of great interest because many LbL applications are found in water. Here, we report upon the nature of this thermal transition as probed using electrochemical impedance spectroscopy (EIS) as a function of assembly salt concentration, film thickness, and outermost layer. EIS reveals that the transition is signified by a structural rearrangement of virtual pores, resulting in increased conductivity and decreased surface coverage of the electrode. Two separate thermal transitions are obtained from changes in the film resistance (Ttr,Rf) and the charge transfer resistance (Ttr,Rct). Only Ttr,Rct is strongly dependent on film thickness, salt concentration, and outermost layer, for which values ranging from 50 to 64 °C were observed. As the assembly salt concentration increases from 0.5 M to 1.0 M NaCl, Ttr,Rct increases by about 10 °C. Below 20 layers, deviations of Ttr,Rct with respect to outermost layer appear, in which PSS-capped LbL films tend to show elevated Ttr,Rct values. These results suggest that extrinsic charge compensation plays a large role in the value of Ttr,Rct in which a large degree of extrinsic charge compensation drives Ttr,Rct towards higher values. On the other hand, Ttr,Rf is largely unaffected by assembly parameters, and closer in value to prior reports via calorimetry and quartz crystal microbalance with dissipation.

 Please wait while we load your content...
Please wait while we load your content...