Thermodynamics of adsorption of ionic surfactants at water/alkane interfaces
Abstract
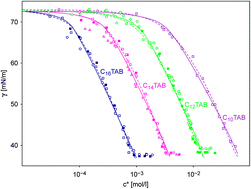
On the basis of experimental data for the homologous series of alkyltrimethylammonium bromides (CnTAB) the equilibrium surface tension isotherms at three types of liquid–fluid interfaces are discussed: solution/air, solution/alkane vapor and solution/liquid alkane interfaces. It is shown that the adsorption characteristics can be described at all three interfaces by the same thermodynamic approach. In the presence of alkane molecules (in the liquid alkane phase or in the alkane vapor phase) the CnTAB adsorption layers can be best described by a co-adsorption of the alkane molecules.

 Please wait while we load your content...
Please wait while we load your content...