The role of the hydrophobic phase in the unique rheological properties of saponin adsorption layers†
Abstract
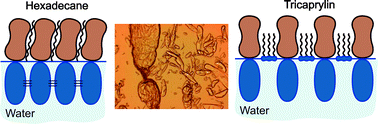
Saponins are a diverse class of natural, plant derived surfactants, with peculiar molecular structure consisting of a hydrophobic scaffold and one or several hydrophilic oligosaccharide chains. Saponins have strong surface activity and are used as natural emulsifiers and foaming agents in food and beverage, pharmaceutical, ore processing, and other industries. Many saponins form adsorption layers at the air–water interface with extremely high surface elasticity and viscosity. The molecular origin of the observed unique interfacial visco-elasticity of saponin adsorption layers is of great interest from both scientific and application viewpoints. In the current study we demonstrate that the hydrophobic phase in contact with water has a very strong effect on the interfacial properties of saponins and that the interfacial elasticity and viscosity of the saponin adsorption layers decrease in the order: air > hexadecane ≫ tricaprylin. The molecular mechanisms behind these trends are analyzed and discussed in the context of the general structure of the surfactant adsorption layers at various nonpolar phase–water interfaces.

 Please wait while we load your content...
Please wait while we load your content...