Rheology and simulation of 2-dimensional clathrin protein network assembly†
Abstract
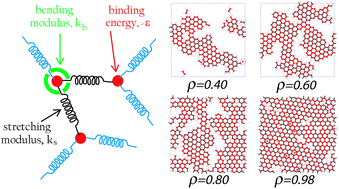
Clathrin is a three-legged protein complex that assembles into lattice structures on the cell membrane and transforms into fullerene-like cages during endocytosis. This dynamic structural flexibility makes clathrin an attractive building block for guided assembly. The assembly dynamics and the mechanical properties of clathrin protein lattices are studied using rheological measurements and theoretical modelling in an effort to better understand two dynamic processes: protein adsorption to the interface and assembly into a network. We find that percolation models for protein network formation are insufficient to describe clathrin network formation, but with Monte Carlo simulations we can describe the dynamics of network formation very well. Insights from this work can be used to design new bio-inspired nano-assembly systems.

 Please wait while we load your content...
Please wait while we load your content...