Characterization of bonds formed between platelet factor 4 and negatively charged drugs using single molecule force spectroscopy†
Abstract
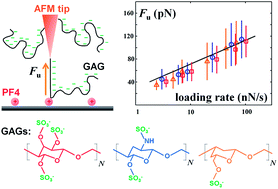
Immunogenicity (i.e., the ability to initiate immune reactions) is one of the major challenges for the development of new drugs, as it may turn the developed drug therapeutically ineffective or cause severe immune-related effects. Using single molecule force spectroscopy, we study rupture forces between the positively charged, endogenous protein platelet factor 4 (PF4; also known as CXC chemokine ligand 4, CXCL4) and the antithrombotic drug heparin and other negatively charged glycosaminoglycans (GAGs), which are known to form immunogenic PF4/GAG-complexes (e.g., heparin and dextran sulfate) as well as non-immunogenic complexes (e.g., chondroitin sulfate A). Our measurements suggest that the average number of sulfate groups per monosaccharide unit (i.e., the degree of sulfation DS) does not affect the unbinding characteristics of single PF4/GAG-bonds (reaction coordinate x0 = 2.2 ± 0.2 Å, energy barrier ΔG ≈ −1 kBT). However, the average number of GAG bonds formed to a single PF4 molecule increases with increasing DS as indicated by a rising frequency of unbinding events, suggesting a multivalent binding scheme between PF4 and GAGs. Our studies show that at least three GAG bonds have to be formed to each PF4 molecule to induce epitope formation on the PF4/GAG-complex to which PF4/GAG-complex specific antibodies bind. Hence, GAG-based drugs that form less than three bonds per PF4 molecule are unlikely to constitute PF4/drug-complexes that are of immunologic relevance.

 Please wait while we load your content...
Please wait while we load your content...