Characterizing protein crystal contacts and their role in crystallization: rubredoxin as a case study
Abstract
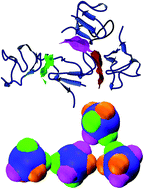
The fields of structural biology and soft matter have independently sought out fundamental principles to rationalize protein crystallization. Yet the conceptual differences and the limited overlap between the two disciplines have thus far prevented a comprehensive understanding of the phenomenon to emerge. We conduct a computational study of proteins from the rubredoxin family that bridges the two fields. Using atomistic simulations, we characterize the protein crystal contacts, and accordingly parameterize patchy particle models. Comparing the phase diagrams of these schematic models with experimental results enables us to critically examine the assumptions behind the two approaches. The study also reveals features of protein–protein interactions that can be leveraged to crystallize proteins more generally.

 Please wait while we load your content...
Please wait while we load your content...