Fe(iii) phytate metallogel as a prototype anhydrous, intermediate temperature proton conductor†
Abstract
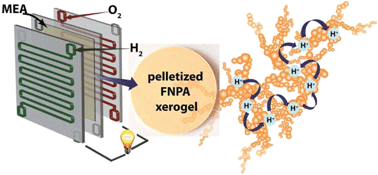
A proton conducting metallogel [FNPA; ferric nitrate (FN)–phytic acid (PA)] is synthesized by immobilizing a protogenic ligand (phytic acid) using iron(III) nitrate in DMF. The xerogel shows high proton conductivity of 2.4 × 10−2 S cm−1 at 120 °C, the best value known among all metal organic materials (MOMs). Marking the first such attempt in MOMs, an electrode made using the xerogel showed a power density of 0.94 mW cm−2 at 0.6 V under dry fuel cell conditions.
- This article is part of the themed collection: Global Energy Challenges: Materials and Nanotechnology

 Please wait while we load your content...
Please wait while we load your content...