The energy blocker inside the power house: mitochondria targeted delivery of 3-bromopyruvate†
Abstract
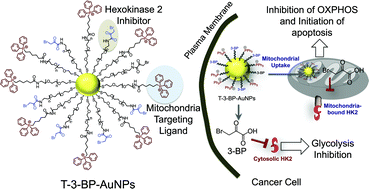
A key hallmark of many aggressive cancers is accelerated glucose metabolism. The enzymes that catalyze the first step of glucose metabolism are hexokinases. Elevated levels of hexokinase 2 (HK2) are found in cancer cells, but only in a limited number of normal tissues. Metabolic reprogramming of cancer cells using the energy blocker 3-bromopyruvate (3-BP), which inhibits HK2, has the potential to provide tumor-specific anticancer agents. However, the unique structural and functional characteristics of mitochondria prohibit selective subcellular targeting of 3-BP to modulate the function of this organelle for therapeutic gain. A mitochondria-targeted gold nanoparticle (T-3-BP-AuNP), decorated with 3-BP and delocalized lipophilic triphenylphosphonium cations to target the mitochondrial membrane potential (Δψm), was developed for delivery of 3-BP to cancer cell mitochondria by taking advantage of the higher Δψm in cancer cells compared to normal cells. In vitro studies demonstrated an enhanced anticancer activity of T-3-BP-AuNPs compared to the non-targeted construct NT-3-BP-AuNP or free 3-BP. The anticancer activity of T-3-BP-AuNPs was further enhanced upon laser irradiation by exciting the surface plasmon resonance band of AuNP and thereby utilizing a combination of 3-BP chemotherapeutic and AuNP photothermal effects. The lower toxicity of T-3-BP-NPs in normal mesenchymal stem cells indicated that these NPs preferentially kill cancer cells. T-3-BP-AuNPs showed an enhanced ability to modulate cancer cell metabolism by inhibiting glycolysis as well as demolishing mitochondrial oxidative phosphorylation. Our findings demonstrate that concerted chemo-photothermal treatment of glycolytic cancer cells with a single NP capable of targeting mitochondria, mediating simultaneous release of a glycolytic inhibitor and photothermal ablation, may have promise as a new anticancer therapy.

 Please wait while we load your content...
Please wait while we load your content...