Asymmetric synthesis of N,O-heterocycles via enantioselective iridium-catalysed intramolecular allylic amidation†
Abstract
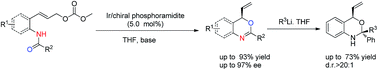
Chiral N,O-heterocycles were synthesized in high yields and excellent enantioselectivity up to 97% ee via iridium-catalysed intramolecular allylic substitution with nucleophilic attack by the amide oxygen atom. The resulting benzoxazine derivatives were further transformed into challenging chiral N,O-ketals bearing both a tertiary and a quaternary center with excellent diastereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...