Hierarchical nanoscale multi-shell Au/CeO2 hollow spheres†
Abstract
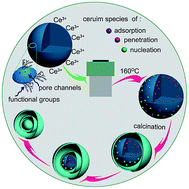
Multi-shell ceria hollow spheres (MSCHSs) with a uniform size of ∼300 nm and controlled shell number up to quadruple were synthesized using carbonaceous spheres as the template in a process combining hydrothermally enhanced metal cation adsorption and tunable calcination. The featured sizes of these MSCHSs are in the hierarchical nanoscale region, including the diameters of the interior and exterior hollow spheres (80–300 nm), the thickness of the shells (∼30 nm), the distance between shells (<100 nm), and the pore size in the shells (∼4 nm), which meant that the as-synthesized MSCHSs possess not only a large specific surface area (∼90 m2 g−1) and narrow mesopore distribution, but also nanosized interconnected chambers. With these structural characteristics, the MSCHSs show fascinating capacities as good hosts for noble metals. Gold nanoparticles with sizes below 5 nm could be loaded on these MSCHSs with a high content and good dispersity to construct effective catalysts, which demonstrated a much improved catalytic performance in the reduction of p-nitrophenol. The optimal values of the reaction rate constant (k) reached up to 0.96 min−1. Moreover, this approach opens up a new way to form nanosized multi-shell structures, especially for those with large cation radii.

 Please wait while we load your content...
Please wait while we load your content...