N2O reduction at a dissymmetric {Cu2S}-containing mixed-valent center†
Abstract
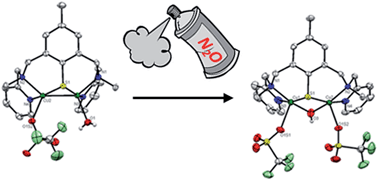
Through our bio-inspired approach toward replicating nitrous oxide reductase (N2Or) activity, treatment of the LMe(MAM)S–S ligand with [Cu(CH3CN)4](OTf) (OTf = trifluoromethanesulfonate ion) leads to the isolation of a new dissymmetric mixed-valent (MV) dicopper(II,I) [2·(H2O)(OTf)]+ containing a {Cu2S} core with labile triflate and water molecules at the copper centers. Whilst [2·(H2O)(OTf)]+ is prone to ligand exchange under particular conditions, a raft of spectroscopic investigations, combined with theoretical calculations demonstrate that its structure is retained in acetone solution. Compared to our previously reported inactive parent complex [1] (Angew. Chem. Int. Ed., 2010, 49 (44), 8249–8252) featuring a symmetric and saturated coordination sphere (N and S atoms from the ligand), [2·(H2O)(OTf)]+ is reactive towards nitrous oxide in acetone. Spectroscopic and theoretical studies combined with kinetic measurements show that exchangeable positions are required for N2O interaction. The isolation of the final product and its characterization by X-ray crystallography as a doubly bridged (μ-thiophenolato)(μ-hydroxo) dicopper(II) species [3·(μ-OH)(OTf)2] help to support the proposed reaction pathway. Implications for N2Or mechanism are discussed.

 Please wait while we load your content...
Please wait while we load your content...