Enantioselective synthesis of tetrahydroquinolines, tetrahydroquinoxalines, and tetrahydroisoquinolines via Pd-Catalyzed alkene carboamination reactions†
Abstract
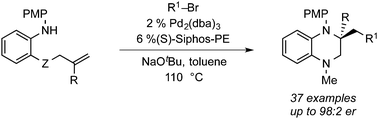
A catalyst composed of Pd2(dba)3 and (S)-Siphos-PE provides excellent results in Pd-catalyzed alkene carboamination reactions between aniline derivatives bearing pendant alkenes and aryl or alkenyl halides. These transformations generate tetrahydroquinolines and tetrahydroquinoxalines that contain quaternary carbon stereocenters with high levels of asymmetric induction. In addition this catalyst also effects the asymmetric synthesis of tetrahydroisoquinolines via related transformations of 2-allylbenzylamines. In contrast to most other approaches to the asymmetric synthesis of these compounds, which frequently involve functional group interconversion or a single C–C or C–N bond-forming event, the carboamination reactions generate both a C–N bond, a C–C bond, and a stereocenter.

 Please wait while we load your content...
Please wait while we load your content...