Bronsted acid-catalyzed skeletal rearrangements in polycyclic conjugated boracycles: a thermal route to a ladder diborole†
Abstract
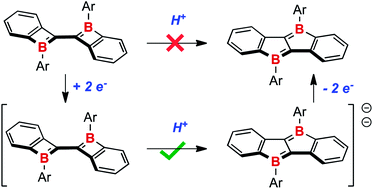
The dibenzodiboracyclobutylidene 1 was recently shown to undergo photochemical rearrangement to the more thermodynamically stable dibenzodiborapentalene 2. In order to discover a more efficient thermal route for this isomerization, the redox chemistry of both 1 and 2 was explored. Radical anions can be formed and characterized by EPR spectroscopy by one electron reduction using potassium graphite. The [Cp*2Co]+ salt of the radical anion of 2 was characterized by X-ray crystallography. The dipotassium salts 1K2 and 2K2 can be prepared by using two equivalents of potassium naphthalenide; both were fully characterized. It was found that smooth rearrangement of 1K2 to 2K2 is mediated by weak Bronsted acids such as tert-butanol or 2,6-dimethylphenol; a mechanism based on similar rearrangements in the isoelectronic neutral all-carbon framework is proposed.

 Please wait while we load your content...
Please wait while we load your content...