Secondary stereocontrolling interactions in chiral Brønsted acid catalysis: study of a Petasis–Ferrier-type rearrangement catalyzed by chiral phosphoric acids†
Abstract
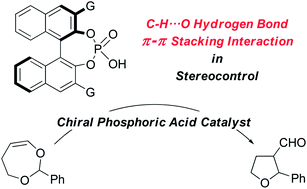
Chiral phosphoric acids have emerged as promising asymmetric Brønsted acid catalysts that harness hydrogen bonding interactions as key stereocontrolling elements. A new approach to chiral phosphoric acid catalysis through ion-pairing interactions between the anionic conjugate base of the catalyst and a cationic electrophile has recently attracted attention. However, the mechanism of stereocontrol through ion-pairing interactions is still elusive. As a probe reaction for studying the stereocontrolling element involved in such catalytic reactions, we investigated the Petasis–Ferrier-type rearrangement of a 7-membered cyclic vinyl acetal catalyzed by chiral phosphoric acids. DFT calculations suggested that non-classical C–H⋯O hydrogen bonds between the catalyst and the substrate play an important role in determining the stereoselectivity. In addition, π–π stacking interactions were found to be a key factor for stereocontrol when using a 9-anthryl group-bearing catalyst.

 Please wait while we load your content...
Please wait while we load your content...