De novo fragment-based design of inhibitors of DXS guided by spin-diffusion-based NMR spectroscopy†
Abstract
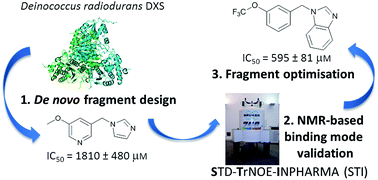
We applied for the first time an innovative ligand-based NMR methodology (STI) to a medicinal-chemistry project aimed at the development of inhibitors for the enzyme 1-deoxy-D-xylulose-5-phosphate synthase (DXS). DXS is the first enzyme of the 2C-methyl-D-erythritol-4-phosphate (MEP) pathway, present in most bacteria (and not in humans) and responsible for the synthesis of the essential isoprenoid precursors. We designed de novo a first generation of fragments, using Deinococcus radiodurans DXS as a model enzyme, targeting the thiamine diphosphate (TDP) pocket of DXS whilst also exploring the putative substrate-binding pocket, where selectivity over other human TDP-dependent enzymes could be gained. The STI methodology – suitable for weak binders – was essential to determine the binding mode in solution of one of the fragments, circumventing the requirement for an X-ray co-crystal structure, which is known to be particularly challenging for this specific enzyme and in general for weak binders. Based on this finding, we carried out fragment growing and optimisation, which led to a three-fold more potent fragment, about as potent as the well-established thiamine analogue deazathiamine. The STI methodology proved therefore its strong potential as a tool to support medicinal-chemistry projects in their early stages, especially when dealing with weak binders.

 Please wait while we load your content...
Please wait while we load your content...