Detecting intracellular translocation of native proteins quantitatively at the single cell level†
Abstract
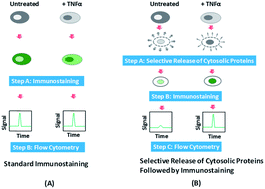
The intracellular localization and movement (i.e. translocation) of proteins are critically correlated with the functions and activation states of these proteins. Simple and accessible detection methods that can rapidly screen a large cell population with single cell resolution have been seriously lacking. In this report, we demonstrate a simple protocol for detecting translocation of native proteins using a common flow cytometer which detects fluorescence intensity without imaging. We sequentially conducted chemical release of cytosolic proteins and fluorescence immunostaining of a targeted protein. The detected fluorescence intensity of cells was shown to be quantitatively correlated to the cytosolic/nuclear localization of the protein. We used our approach to detect the translocation of native NF-κB (an important transcription factor) at its native expression level and examine the temporal dynamics in the process. The incorporation of fluorescence immunostaining makes our approach compatible with the analysis of cell samples from lab animals and patients. Our method will dramatically lower the technological hurdle for studying subcellular localization of proteins.

 Please wait while we load your content...
Please wait while we load your content...