Millisecond lifetime imaging with a europium complex using a commercial confocal microscope under one or two-photon excitation†
Abstract
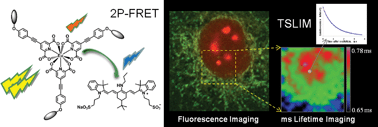
The long luminescence lifetime of lanthanide based bioprobes is a great advantage for their specific detection in autofluorescent or labelled cells and tissues. It is also a valuable tool for sensing the physicochemical microenvironment and molecular interactions by Förster resonance energy transfer (FRET). However, standard confocal and multiphoton laser scanning microscopes are not adapted for imaging with such temporal resolution, because the typical pixel dwell time is too short compared to the luminescence lifetime. We show that the rapid sampling rate and laser control of a usual confocal microscope can instead be used for precise measurement of long lifetime decays (μs to ms range). Furthermore, both raster- and line-scanning microscopes can specifically detect long luminescence signals in the time-gated mode by shifting the pinhole or the confocal slit in the lagging direction. We characterized the subcellular localization and accurately measured the millisecond luminescence lifetimes of the benchmark two-photon europium probe [Na]3[EuL1G3], and specifically imaged this label in the presence of short-lived fluorescent species. Fine variations of the luminescence lifetime of this lanthanide complex were revealed and mapped in cells in the presence of a FRET acceptor, allowing quantification of the FRET efficiency independently of donor concentration. These results demonstrate a high and yet unexploited potential of quantitative confocal and multiphoton microscopy for time-gated and lifetime imaging of lanthanide-based biological sensors.

 Please wait while we load your content...
Please wait while we load your content...