Enantiomeric differentiation of aromatic amino acids using traveling wave ion mobility-mass spectrometry†
Abstract
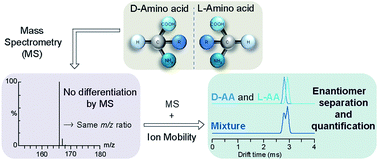
The present work describes the first differentiation of enantiomers using the coupling of traveling wave ion mobility and mass spectrometry (TWIM-MS). This study was carried out on amino acids, the building blocks of proteins, which together with nucleotides, polysaccharides or lipids, are the main constituents of all living organisms. Herein, the enantiomers of aromatic amino acids (AA) such as phenylalanine, tryptophan and tyrosine are differentiated by TWIM-MS through their cationisation with copper(II) and multimer formation with D-proline (Pro) as a chiral reference compound. This methodology can be considered as an alternative approach to conventional methods for the separation of enantiomers. Moreover, quantification of the enantiomers can be performed easily and quickly using TWIM-MS analysis of the ionic complex [(DPro)2+D/LAA+CuII–H]+.

 Please wait while we load your content...
Please wait while we load your content...