Enantioselective direct α-alkylation of cyclic ketones by means of photo-organocatalysis†
Abstract
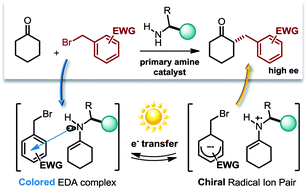
We report here the first asymmetric catalytic alkylation of unmodified ketones with alkyl halides. This metal-free approach, which requires light in order to proceed, provides a rare example of highly enantioselective photochemical catalytic processes. An easily available cinchona-based primary amine catalyst guides both the stereoselectivity-defining event and, through the transient formation of photon-absorbing chiral electron donor–acceptor complexes, the photo-activation of the substrates.

 Please wait while we load your content...
Please wait while we load your content...