Total syntheses of indolactam alkaloids (−)-indolactam V, (−)-pendolmycin, (−)-lyngbyatoxin A, and (−)-teleocidin A-2†
Abstract
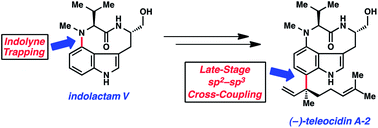
We report the total syntheses of (−)-indolactam V and the C7-substituted indolactam alkaloids (−)-pendolmycin, (−)-lyngbyatoxin A, and (−)-teleocidin A-2. The strategy for preparing indolactam V relies on a distortion-controlled indolyne functionalization reaction to establish the C4–N linkage, in addition to an intramolecular conjugate addition to build the conformationally-flexible nine-membered ring. The total synthesis of indolactam V then sets the stage for the divergent synthesis of the other targeted alkaloids. Specifically, late-stage sp2–sp3 cross-couplings on an indolactam V derivative are used to introduce the key C7 substituents and the necessary quaternary carbons. These challenging couplings, in addition to other delicate manipulations, all proceed in the presence of a basic tertiary amine, an unprotected secondary amide, and an unprotected indole. Thus, our approach not only enables the enantiospecific total syntheses of four indolactam alkaloids, but also serves as a platform for probing complexity-generating and chemoselective transformations in the context of alkaloid total synthesis.

 Please wait while we load your content...
Please wait while we load your content...