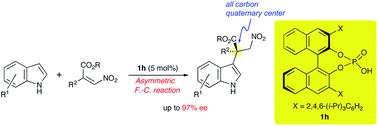
Stereoselective construction of all-carbon quaternary center by means of chiral phosphoric acid: highly enantioselective Friedel–Crafts reaction of indoles with β,β-disubstituted nitroalkenes†
Abstract
We describe herein a chiral phosphoric acid catalyzed Friedel–Crafts reaction of indoles with β-alkoxycarbonyl-β-disubstituted nitroalkenes. A wide variety of substrates participated in this reaction to afford indoles having all-carbon quaternary centers with excellent selectivities (up to 97% ee). Further investigation suggested that the olefin geometry and the employment of NH-indole derivatives are responsible for both reactivity and selectivity.

 Please wait while we load your content...
Please wait while we load your content...