Non-directed allylic C–H acetoxylation in the presence of Lewis basic heterocycles†
Abstract
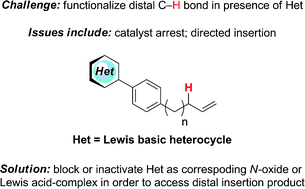
We outline a strategy to enable non-directed Pd(II)-catalyzed C–H functionalization in the presence of Lewis basic heterocycles. In a high-throughput screen of two Pd-catalyzed C–H acetoxylation reactions, the addition of a variety of N-containing heterocycles is found to cause low product conversion. A pyridine-containing test substrate is selected as representative of heterocyclic scaffolds that are hypothesized to cause catalyst arrest. We pursue two approaches in parallel that allow product conversion in this representative system: Lewis acids are found to be effective in situ blocking groups for the Lewis basic site, and a pre-formed pyridine N-oxide is shown to enable a high yield of allylic C–H acetoxylation. Computational studies with density functional theory (M06) of the binding affinities of selected heterocycles to Pd(OAc)2 provide an inverse correlation of the computed heterocycle–Pd(OAc)2 binding affinities with the experimental conversions to products. Additionally, 1H NMR binding studies provide experimental support for the theoretical calculations.

 Please wait while we load your content...
Please wait while we load your content...