Synthesis of conjugation-ready zwitterionic oligosaccharides by chemoselective thioglycoside activation†
Abstract
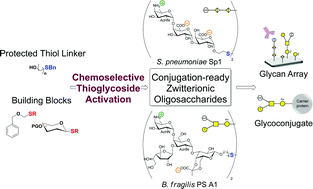
Commensal bacteria are ubiquitous inhabitants of mucosal surfaces and play an important role in promoting the maturation of the mammalian immune system. Zwitterionic polysaccharides (ZPSs) are found on the surface of certain commensal bacteria and exhibit important immunomodulatory activity. ZPSs are the first known carbohydrate antigens to induce an immune response by a T cell-dependent pathway. To understand the mechanism of their immunomodulatory activity, structurally-defined ZPS probes are needed. Here, we report the first total syntheses of conjugation-ready repeating units of the two most prominent ZPSs, S. pneumoniae Sp1 (1) and B. fragilis PS A1 (2), and their immunological characterization after conjugation to reporter moieties. The introduction of a thioether-containing linker at an early stage of the synthesis called for establishing a method to chemoselectively activate thioglycosides in the presence of benzylthioethers. After oligosaccharide assembly, the same mild activation conditions were used in a novel way to introduce a benzyloxymethyl ether to cap the base-labile AAT residue, which allowed for completion of the syntheses. The appended thiol linkers enabled the conjugation of oligosaccharides 1 and 2 to glycan array and carrier protein moieties. Glycan array analysis revealed recognition of synthetic Sp1, but not PS A1, by antiserum against the native polysaccharide, demonstrating the applicability of conjugation-ready ZPS probes in biochemical settings. Further studies will give insight into the immunomodulatory properties of ZPSs.

 Please wait while we load your content...
Please wait while we load your content...