Development of a route to chiral epidithiodioxopiperazine moieties and application to the asymmetric synthesis of (+)-hyalodendrin†
Abstract
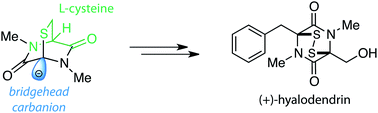
The epidithiodioxopiperazine skeleton is found in a variety of biologically active compounds. Despite numerous attempts at constructing this highly functionalized structure within a bicyclo[2.2.2]octane skeleton, asymmetric synthesis of this unique functionality remains problematic. Our synthetic studies have led to the development of efficient methods for the asymmetric preparation of an epidithiodioxopiperazine skeleton, which was successfully applied to the first asymmetric synthesis of (+)-hyalodendrin.

 Please wait while we load your content...
Please wait while we load your content...