Sandmeyer trifluoromethylthiolation of arenediazonium salts with sodium thiocyanate and Ruppert–Prakash reagent†
Abstract
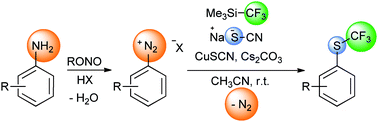
In the presence of copper thiocyanate, sodium thiocyanate and the inexpensive, easy-to-use trifluoromethylating reagent Me3Si–CF3, diazonium salts are smoothly converted into the corresponding aryl trifluoromethyl thioethers. Combined with diazotisation, this convenient and inexpensive method allows the straightforward synthesis of aryl or heteroaryl trifluoromethyl thioethers from the corresponding anilines.

 Please wait while we load your content...
Please wait while we load your content...