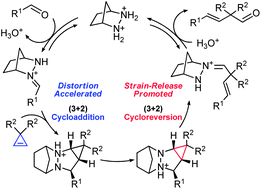
Distortion-accelerated cycloadditions and strain-release-promoted cycloreversions in the organocatalytic carbonyl-olefin metathesis†
Abstract
The mechanism of hydrazine-catalyzed carbonyl-olefin metathesis relying on a novel (3 + 2) strategy is studied by density functional theory (DFT) calculations. The origins of the special reactivity of cyclopropene in this transformation are revealed, and the reactivities of different alkenes in the (3 + 2) cycloadditions and cycloreversions are compared. It is found that the ease of distortion of reactants accelerates cycloadditions, and that the strain release is the controlling factor for cycloreversions.

 Please wait while we load your content...
Please wait while we load your content...