Iridium-catalyzed diborylation of benzylic C–H bonds directed by a hydrosilyl group: synthesis of 1,1-benzyldiboronate esters†
Abstract
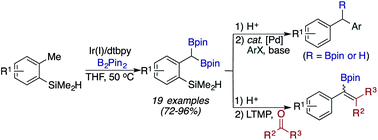
We describe a regioselective diborylation of primary benzylic C–H bonds catalyzed by [Ir(COD)OMe]2 and 4,4′-di-tert-butyl-2,2′-bipyridine (dtbpy). The hydrosilyl group acts as a traceless directing group, providing access to a range of 1,1-benzyldiboronate esters in good yields. Transformations of the 1,1-benzyldiboronate ester products include chemoselective Suzuki–Miyaura cross-couplings and synthesis of tetrasubstituted alkenyl boronate esters.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners

 Please wait while we load your content...
Please wait while we load your content...