Catalytic enantioselective synthesis of 2-(2-hydroxyethyl)indole scaffolds via consecutive intramolecular amido-cupration of allenes and asymmetric addition of carbonyl compounds†
Abstract
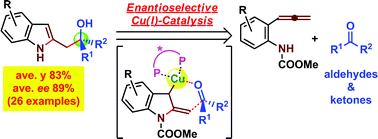
A catalytic enantioselective method for the synthesis of 2-(2-hydroxyethyl)indole scaffolds was developed. The process includes catalytic intramolecular amido-cupration of an allene to generate a novel allylcopper species, followed by asymmetric addition of the thus-generated chiral nucleophile to aldehydes and ketones. This is the first example of catalytic indole formation coupled with asymmetric C–C bond formation via in situ generation of a reactive chiral allylcopper species.

 Please wait while we load your content...
Please wait while we load your content...