Organocatalytic C–H hydroxylation with Oxone® enabled by an aqueous fluoroalcohol solvent system†
Abstract
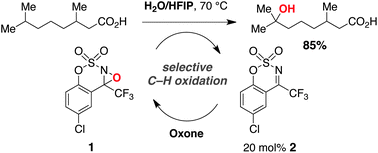
Selective hydroxylation of 3° and benzylic C–H bonds is made possible using a non-metal-based catalyst system, Oxone as the terminal oxidant, and an aqueous fluoroalcohol solvent mixture. The choice of solvent is uniquely effective for this process, but seemingly at odds with our finding that H2O promotes reduction of the oxaziridine intermediate. Our studies suggest that the hydroxylation reaction is occurring within a fluoroalcohol microdroplet, which both concentrates the reactants and mitigates the deleterious impact of H2O on oxaziridine stability. These discoveries have led to demonstrable improvements in the organocatalytic oxygenation of hydrocarbon substrates and, for the first time, the successful use of Oxone with this catalyst system. Reactions generally afford product and unreacted starting material in near quantitative amounts and display outstanding selectivity for 3° and benzylic C–H bond oxidation.
- This article is part of the themed collection: C-H Functionalization

 Please wait while we load your content...
Please wait while we load your content...