Torsional control of stereoselectivities in electrophilic additions and cycloadditions to alkenes
Abstract
Torsional effects control the π-facial stereoselectivities of a variety of synthetically important organic reactions. This review surveys theoretical calculations that have led to the understanding of the influence of the torsional effects on several types of stereoselective organic reactions, especially electrophilic additions and cycloadditions to alkenes.
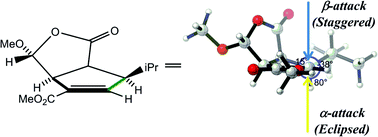

 Please wait while we load your content...
Please wait while we load your content...