Abstract
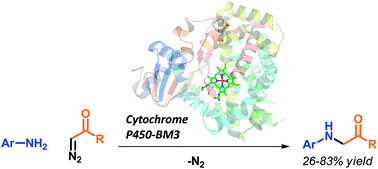
Expanding nature's catalytic repertoire to include reactions important in synthetic chemistry will open new opportunities for ‘green’ chemistry and biosynthesis. We demonstrate the first enzyme-catalyzed insertion of carbenoids into N–H bonds. This type of bond disconnection, which has no counterpart in nature, can be mediated by variants of the cytochrome P450 from Bacillus megaterium. The N–H insertion reaction takes place in water, provides the desired products in 26–83% yield, forms the single addition product exclusively, and does not require slow addition of the diazo component.
- This article is part of the themed collection: 7th EuCheMS Chemistry Congress – Molecular frontiers and global challenges

 Please wait while we load your content...
Please wait while we load your content...