The reorganization energy of intermolecular hole hopping between dyes anchored to surfaces†
Abstract
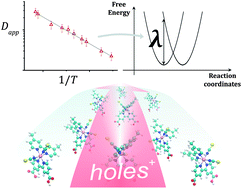
We measured the rate of hole hopping between dye molecules on titanium dioxide nanocrystals using cyclic voltammetry. Dyes commonly used in the field of dye sensitized solar cells exhibited efficient intermolecular charge transport, showing apparent diffusion coefficient values between 10−8 up to over 10−7 cm2 s−1 at room temperature. From temperature dependent measurements, we observed that hole transport across dye monolayers is a thermally activated process with Arrhenius activation energies between about 170 and 370 meV depending on the dye. Analysis of the data in terms of non-adiabatic Marcus theory of charge transfer enabled the estimation of the reorganization energy (740–1540 meV) and of an effective electronic coupling for the different systems. The measured reorganization energies show reasonable agreement with values obtained from density functional theory based calculations, validating our computational approach. Finally, we interpret the experimental and calculated data with reference to the chemical structure of the dyes and to the packing of the dyes on the surface of the TiO2 and suggest that delocalization of the HOMO and rigidity of the conjugated molecular structure result respectively in lower outer and inner sphere reorganization energies.

 Please wait while we load your content...
Please wait while we load your content...