Biosynthesis of thiomarinol A and related metabolites of Pseudoalteromonas sp. SANK 73390†
Abstract
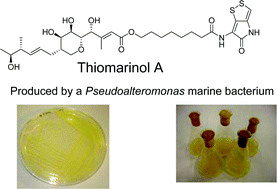
The biosynthesis of the mixed PKS-FAS-NRPS hybrid antibiotic thiomarinol A was investigated using feeding studies to both wild type and mutant strains of the marine bacterium Pseudoalteromonas. Particularly interesting features of the pathway include assembly of the 8-hydroxyoctanoic acid side-chain via chain extension of a C4-precursor (4-hydroxybutyrate), and construction of the pyrrothine unit from cysteine via (HolA-D, F-H) prior to intact incorporation into thiomarinol (catalysed by TmlU). A series of thiomarinol-related and other minor metabolites have been isolated from wild-type and mutant strains. The results of these investigations are rationalised in terms of the overall biosynthetic pathway.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners

 Please wait while we load your content...
Please wait while we load your content...