Photoredox activation and anion binding catalysis in the dual catalytic enantioselective synthesis of β-amino esters†
Abstract
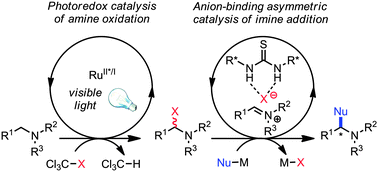
The enantioselective oxidative C–H functionalization of tetrahydroisoquinoline derivatives is achieved through the merger of photoredox and asymmetric anion-binding catalysis. This combination of two distinct catalysis concepts introduces a potentially general approach to asymmetric transformations in oxidative photocatalysis.

 Please wait while we load your content...
Please wait while we load your content...