High CO2/N2/O2/CO separation in a chemically robust porous coordination polymer with low binding energy†
Abstract
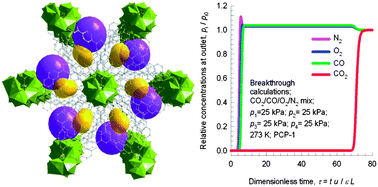
Porous coordination polymers (PCPs), constructed from organic linkers and metal ions, can provide special pore environments for selective CO2 capture. Although many PCPs have been reported, a rational design for identifying PCPs that adsorb CO2 molecules with a low binding energy, high separation ability and high chemical stability remains a great challenge. Here, we propose and validate, experimentally and computationally, a new PCP, [La(BTN)DMF]·guest (PCP-1⊃guest), that has a large aromatic organic surface and a low binding energy for high CO2 separation from four-gas mixtures (CO2–N2–O2–CO) at ambient temperature. In addition, it shows good water and chemical stability; in particular, it is stable from pH = 2 to 12 at 100 °C, which is unprecedented for carboxylate-based PCPs.

 Please wait while we load your content...
Please wait while we load your content...