Chemoselective sulfenylation and peptide ligation at tryptophan†
Abstract
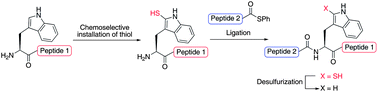
Peptide ligation–desulfurization chemistry at 2-thiol tryptophan (Trp) is described for the first time. Installation of a thiol auxiliary was achieved through late-stage chemoselective sulfenylation chemistry at the 2-position of the indole ring of Trp either in solution or on solid support, thus abrogating the need for the preparation of a pre-formed thiolated amino acid. Peptides possessing the 2-thiol Trp functionality on the N-terminus were shown to facilitate high yielding ligation reactions with a variety of C-terminal peptide thiophenyl thioesters. Efficient removal of the 2-thiol Trp auxiliary following the ligation reactions was achieved via reductive desulfurization and provided native peptide products in excellent yields. The utility of the methodology was demonstrated in the synthesis of a glycosylated fragment of the N-terminal extracellular domain of the chemokine receptor CXCR1.

 Please wait while we load your content...
Please wait while we load your content...