Review of the bulk and surface chemistry of iron in atmospherically relevant systems containing humic-like substances
Abstract
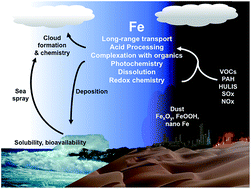
As the fourth most abundant element by mass in the Earth's crust, iron is ubiquitous and its chemistry is rich and interdisciplinary in nature. This review synthesizes the current state of knowledge of iron chemistry in multicomponent atmospheric aerosols. This knowledge is also applicable to other atmospherically relevant systems that include iron-containing anthropogenic nanodust, ocean surfaces and buildings. Because of the abundance of humic-like substances in these systems, this review focuses on the chemistry of these substances with iron compounds. Findings from field measurements and laboratory studies are summarized to highlight the major themes in the chemical reactivity of iron, which varies depending on the solubility, the redox conditions, the absence and presence of UV-visible light and reactive oxygen species, the pH and the temperature. This review also highlights the key differences between the bulk and surface chemistry of iron-containing materials, which varies considerably because of the structure of the interfacial water and the solvent cage effect. Additional laboratory, field and modelling studies are needed to better understand the contributions of transition metal chemistry to the formation of secondary organic aerosols and also the chemistry, uptake and release of trace gas phase species. This information will improve the predictive power of models that incorporate aerosol chemistry and physics.

 Please wait while we load your content...
Please wait while we load your content...