Metal free chemoselective reduction of α-keto amides using TBAF as catalyst†
Abstract
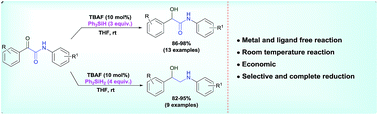
The metal and ligand free chemoselective reduction of the keto group and complete reduction of the both keto and amide groups of α-keto amide with hydrosilanes using tetrabutylammoniumflouride (TBAF) as catalyst have been accomplished. This methodology affords an efficient and economic route for the synthesis of biologically important α-hydroxy amides and β-amino alcohols. The other important advantage of this TBAF catalyst is chemoselective reduction of ketones to corresponding alcohols in the presence of several other sensitive functional groups.

 Please wait while we load your content...
Please wait while we load your content...