Stability indicating RP-HPLC method for the determination of flubendazole in pharmaceutical dosage forms
Abstract
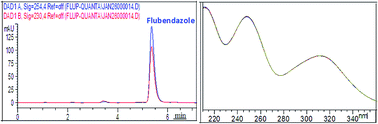
This paper presents a stability study of the drug flubendazole (FLUB) – a benzimidazole carbamate derivative with anthelmintic activity. In order to investigate the stability of the drug, FLUB was subjected to stress degradation under different conditions, as recommended by the International Conference on Harmonization (ICH). A validated HPLC-photodiode array detector (DAD) method was established to resolve FLUB from all its degradation products obtained under acidic, basic, neutral, oxidation, photo-degradation, and thermal conditions, and also from its suspension additives. Chromatographic separation was achieved on a ZORBAX Eclipse Plus C18 column in an isocratic mode of elution using a mixture of water : acetonitrile (50 : 50, v/v) as a mobile phase, at a flow rate of 1 mL min−1, where FLUB was well resolved from all its degradation products at a tR of 5.35 min. The thermostated column compartment (TCC) was adjusted to 25 °C and the effluent was monitored by a photo-diode array detector at 254 nm, and FLUB was found to be linear in the range of 0.5–10 μg mL−1. The method was validated in terms of accuracy, precision, specificity, robustness, and ruggedness, as per USP guidelines. It was successfully applied to quantify FLUB in bulk powder and in pharmaceutical formulations with complete resolution between FLUB and its suspension additives. Statistical analyses between the suggested method and the official HPLC method using student's-t and F-ratio tests reveal that the suggested method is as accurate and precise as the reported one.

 Please wait while we load your content...
Please wait while we load your content...