A carboxylic acid-functionalized coumarin-hemicyanine fluorescent dye and its application to construct a fluorescent probe for selective detection of cysteine over homocysteine and glutathione†
Abstract
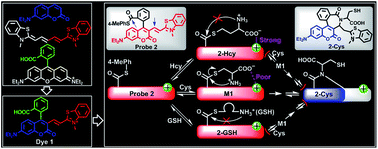
A carboxylic acid-functionalized coumarin-hemicyanine near-infrared (NIR) dye 1 was exploited, which possesses good water solubility (more than 50 μM) and favorable photophysical properties, especially a large Stokes shift (around 90 nm), and has been proved to be a suitable imaging agent for targeting mitochondria. With the dye platform, fluorescent probe 2, a thioester derivative of 1, was constructed for biothiols. Probe 2 can react with cysteine (Cys) via the native-chemical-ligation (NCL) and cyclization cascade reactions to lead to coumarin 2-Cys. However, the reaction of 2 with homocysteine (Hcy) or glutathione (GSH) only stays at the stage of the initial transthioesterification reaction, producing coumarin-hemicyanines 2-Hcy or 2-GSH, due to an electrostatic interaction in 2-Hcy and an unstable macrocyclic transition state in 2-GSH, both inhibiting their subsequent S,N-acyl shift. Given the distinct photophysical properties between 2-Cys and 2-Hcy (or 2-GSH), probe 2 could highly selectively discriminate Cys from Hcy/GSH. Even in the presence of Hcy or GSH, probe 2 still works well for Cys due to the reversible transthioesterification and the irreversible S,N-acyl shift in the NCL reaction. The cell imaging assays revealed that probe 2 is cell permeable and could selectively image Cys in living cells.

 Please wait while we load your content...
Please wait while we load your content...