Phenylalanine iminoboronates as new phenylalanine hydroxylase modulators†
Abstract
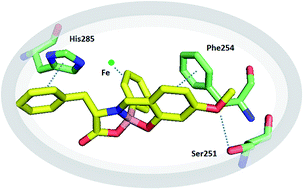
Herein we report the discovery of new modulators of human phenylalanine hydroxylase (hPAH) inspired by the structure of its substrate and regulator L-phenylalanine. These new hPAH modulators were simply prepared in good-to-excellent yields and excellent diastereoselectivities, based on a boron promoted assembly of L-phenylalanine, salicylaldehyde and aryl boronic acids. Iminoboronate 8, prepared with L-phenylalanine, para-methoxy-salicylaldehyde and phenyl boronic acid, was identified as the most efficient hPAH modulator, with an apparent binding affinity nearly identical to the natural allosteric activator L-phenylalanine.

 Please wait while we load your content...
Please wait while we load your content...