Control of a two-dimensional molecular structure by cooperative halogen and hydrogen bonds
Abstract
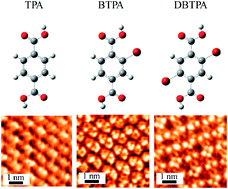
The cooperative effect of hydrogen and halogen bonds on the two-dimensional (2D) molecular arrangement on highly oriented pyrolytic graphite (HOPG) was studied by scanning tunneling microscopy. The terephthalic acid (TPA) molecule, which has two carboxyl groups attached at the para positions of a benzene ring, formed a one-dimensional (1D) linear non-covalent network structure on HOPG by hydrogen bonds between the carboxyl groups of neighboring molecules. However, unlike the TPA molecule, Br substituted TPA molecules were found to form different non-covalent network structures. Owing to Br⋯O halogen and hydrogen bonds, bromo-substituted TPA (2-bromoterephthalic acid) formed a 1D ladder-like non-covalent network structure, whereas dibromo-substituted TPA (2,5-dibromoterephthalic acid) formed a 2D non-covalent lattice network on HOPG. These results strongly indicate that Br⋯O halogen bonds significantly contribute to determine the molecular assembly as well as hydrogen bonds for molecules containing bromine groups and hydrogen bonding groups. These results provided deep fundamental insight into the cooperative effect of halogen and hydrogen bonds in 2D molecular assemblies. In addition, we have demonstrated that these interactive bonds are promising for the precise design of 2D molecular architectures.

 Please wait while we load your content...
Please wait while we load your content...