Phase and shape dependent electrochemical properties of BiPO4 by PVP assisted hydrothermal method for pseudocapacitors†
Abstract
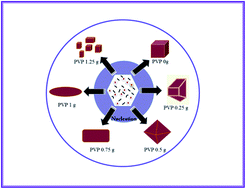
BiPO4 materials with different shapes are obtained under hydrothermal conditions by simply varying the concentration of PVP (polyvinylpyrrolidone). The prepared materials are characterized using various techniques such as X-Ray Diffraction (XRD), Thermo Gravimetric/Differential Scanning Calorimetry (TG/DSC), Fourier Transform Infrared (FT-IR) spectra, Field Emission Scanning Electron Microscopy (FE-SEM) and Transmission Electron Microscopy (TEM). The XRD with Rietveld analysis infers the formation of a monazite-type monoclinic BiPO4 structure. A transformation from monazite-type into trigonal BiPO4 phase is observed upon increasing the PVP concentration. The FE-SEM images reveal the change of shape from irregular cubic structure to octahedral to spindle upon increasing the concentration of PVP from 0.25 g to 1.0 g and thereafter the shape begins to disintegrate (1.25 g). Similarly, the effect of reaction time on the shape of BiPO4 is also studied. The electrochemical performance of pristine BiPO4 electrode is studied in various aqueous electrolytes such as 1 M LiOH, 1 M NaOH, 1 M KOH, 0.5 M K2SO4 and 1 M KNO3. Overall, the pristine BiPO4 provides a maximum specific capacitance in 1 M KOH electrolyte and a specific capacitance of 89 F g−1 is obtained at 5 mA cm−2. At low PVP concentration, there is an increase in specific capacitance whereas it decreases at high concentrations (1.0 g and 1.25 g). The monazite-type monoclinic structure with rod shape particles exhibits a high specific capacitance (167 F g−1 at 5 mA cm−2) and cycle life compared with other morphologies. The effective role of crystal phase and shape on the electrochemical performance of BiPO4 is elucidated.

 Please wait while we load your content...
Please wait while we load your content...